Peter Jung, Peter Pham, Jack Pirie, Jack Plant and Russell Sumarno
- Community Partner: FMRK Diagnostic Technologies
- Degree: Bachelor of Applied Science
- Program:
- Campus: Vancouver
The challenge
Our client, FMRK Diagnostic Technologies, develops innovative diagnostic tests that can be used in community health-care settings. They asked us to develop a point-of-care device that would automate and speed up the process of identifying the most effective antibiotic to treat urinary tract infections.
In current practice, patients provide a urine sample that is sent to an off-site lab or hospital lab for an antibiotic susceptibility test to determine which treatment option is most effective for the particular infection. This process can take up to two days or even longer in remote communities. Because of the lengthy turnaround time, health-care practitioners often prescribe broad spectrum antibiotics before they have received the test results, which can lead to antibiotic resistance.
We were asked to develop an easy-to-use device that could be used at the point of care – such as in a long-term care home or in a local pharmacy.
The device should allow for a fully automated screening process to test the sample and identify the most appropriate narrow spectrum antibiotic based on the bacteria present.
Our process and the challenges we faced
This project integrates multiple areas of engineering – such as mechanical, electrical and automation – as well as wet lab skills to conduct the antibiotic susceptibility tests. Fortunately, our team has a wide range of skill sets based on the courses we’ve taken and our experiences in co-op and design teams. To take advantage of our areas of expertise, we split the work into modules with each person taking responsibility for one area.
We drew inspiration from many sources – from a 1990s BMW cup tray for the mechanics for our microfluidic chip tray to actuation mechanisms we’ve worked with on other projects.
While our device relies on existing technologies, the innovation is how we have combined them into a cohesive product.
We learned a lot over the course of this project, from how to do assays correctly so we could validate the proof of concept for the sterile transfer method to using the animation tools in CAD to deliver a critical function prototype, one of our early deliverables.
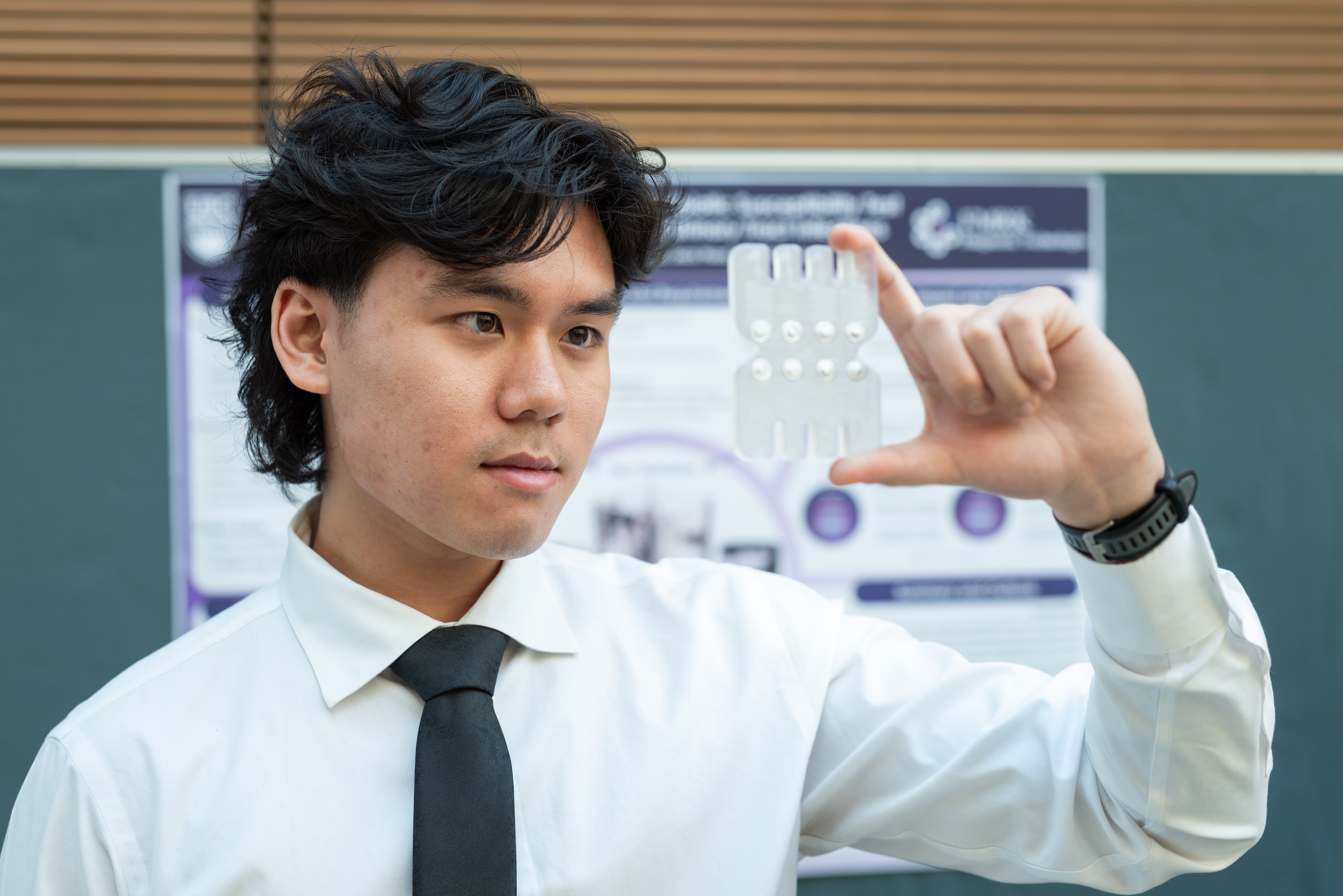
Our project’s future
We’ve shown proof of concept that this device is feasible and works. There are many additional technical issues to resolve, including optimizing the optical system, finetuning the algorithm for the antibiotic susceptibility testing, and developing the cleaning and waste management system.
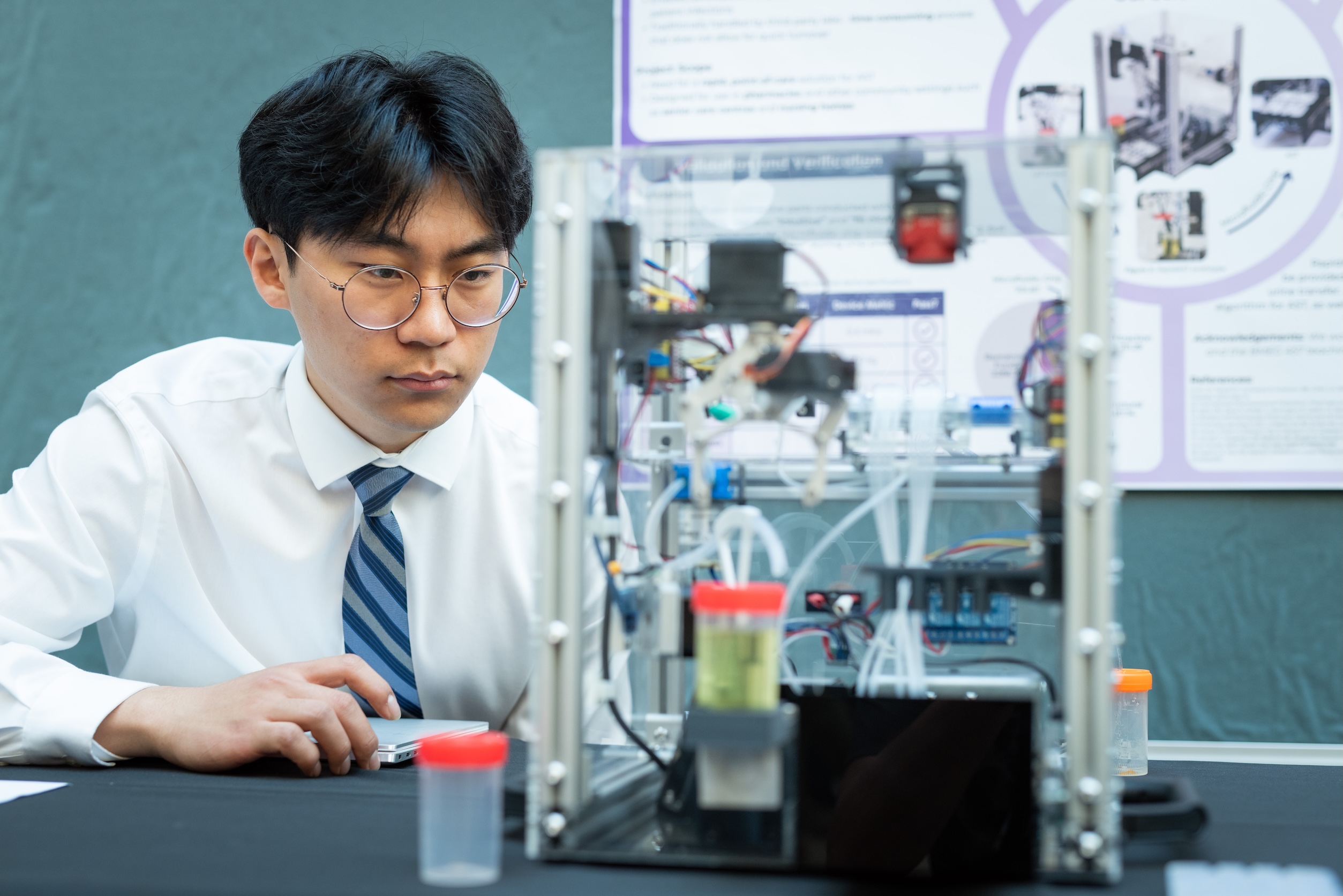
Once a working prototype is in place, there are many other steps to complete before the device would be ready for market. As our client, Faisal Khan, the founder and CEO of FMRK, explains, “These guys understand the science and engineering but it’s my job to offer guidance on the regulator pathways, intellectual property considerations, reimbursement, and market opportunities to make sure the solution they develop is tailored to those parameters.”
This device could make a significant difference in empowering health-care providers to quickly identify the narrow spectrum antibiotic most effective at treating urinary tract infections.
It represents an easy-to-use solution that could be implemented in pharmacies, long-term care homes and other sites, alleviating the burden on existing lab facilities and helping patients recover much faster from urinary tract infections.